Effect of base (NaOH) and acid (HCl) additions on changes in buffering... | Download Scientific Diagram

Selective Focus of Sodium Hydroxide Base and Sulfuric Acid Solution in Brown Glass and Plastic Bottle Stock Photo - Image of base, hydroxide: 195465080
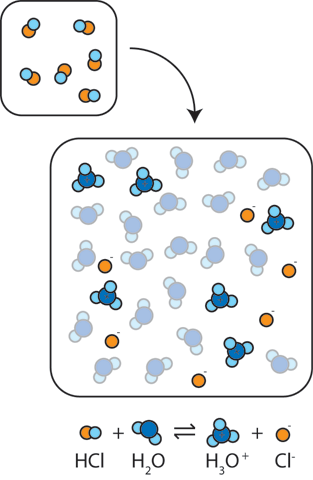
Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution? | Socratic

Sodium hydroxide (NaOH) is classified as a strong base. For every mole of sodium hydroxide added to a large volume of water, one mole of what ion enters the solution?
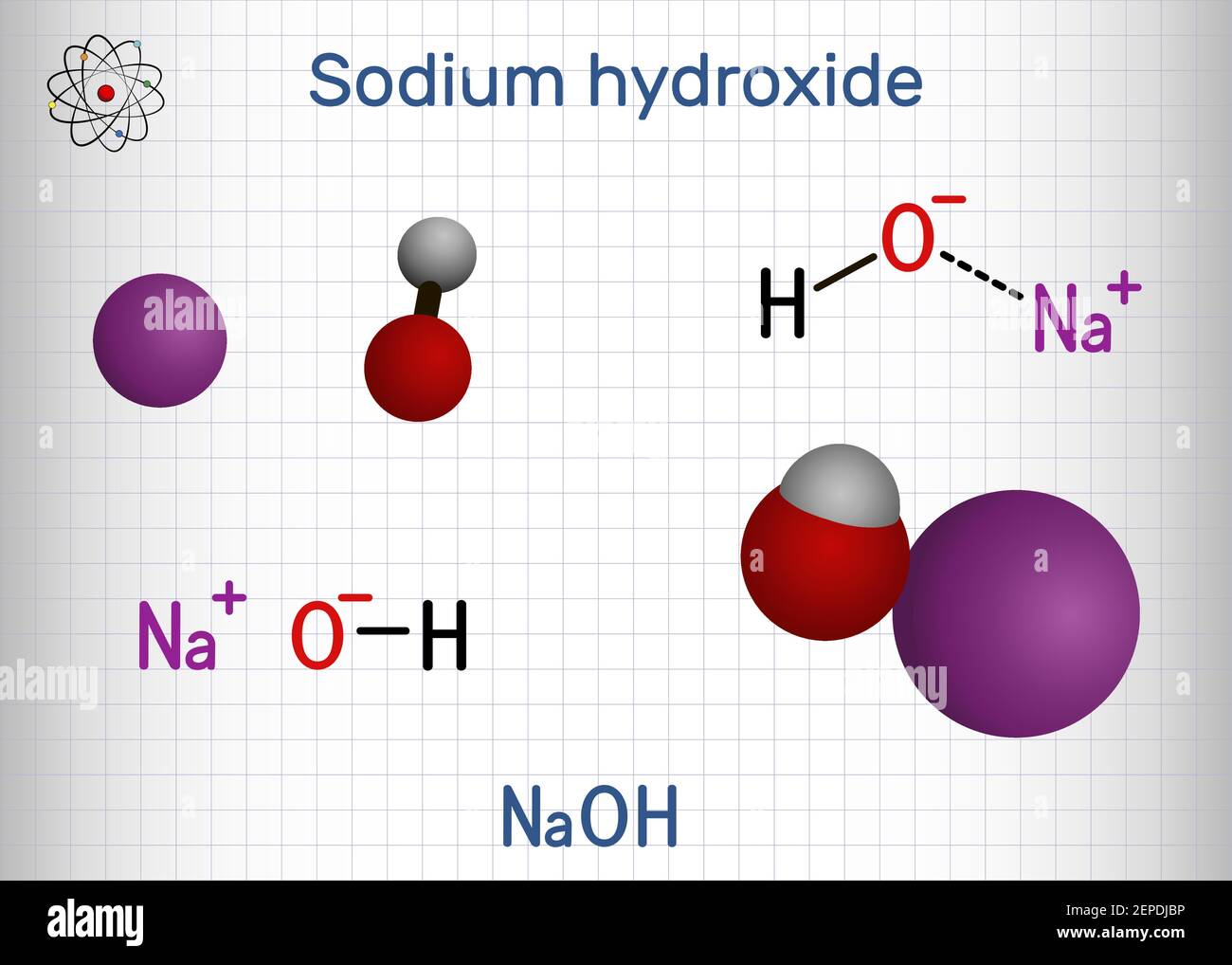
Sodium hydroxide, caustic soda, lye molecule. NaOH is highly caustic base and alkali, ionic compound. Structural chemical formula and molecule model Stock Vector Image & Art - Alamy

Selective Focus of Sodium Hydroxide Base and Sulfuric Acid Solution in Brown Glass and Plastic Bottle Stock Image - Image of harmful, class: 195465085





:max_bytes(150000):strip_icc()/prepare-sodium-hydroxide-or-naoh-solution-608150_FINAL-696b52d6f90b4b1383ec8f95db73a1f3.png)
-in-water-01.jpg)